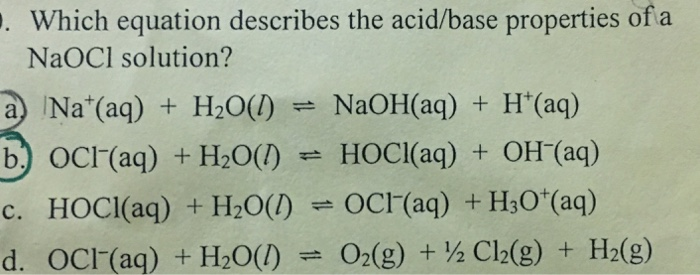Effect of sodium hypochlorite on adhesive charactersitics of dentin: A systematic review of laboratory-based testing - ScienceDirect

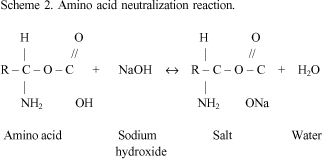
SOLVED: Match the following salts with the acid-base neutralization reactants that would produce them: A. NaClO4 = HCl + NaOH B. NaOCl = HCl + NaOH C. Ca(OCl)2 = HCl + Ca(OH)2
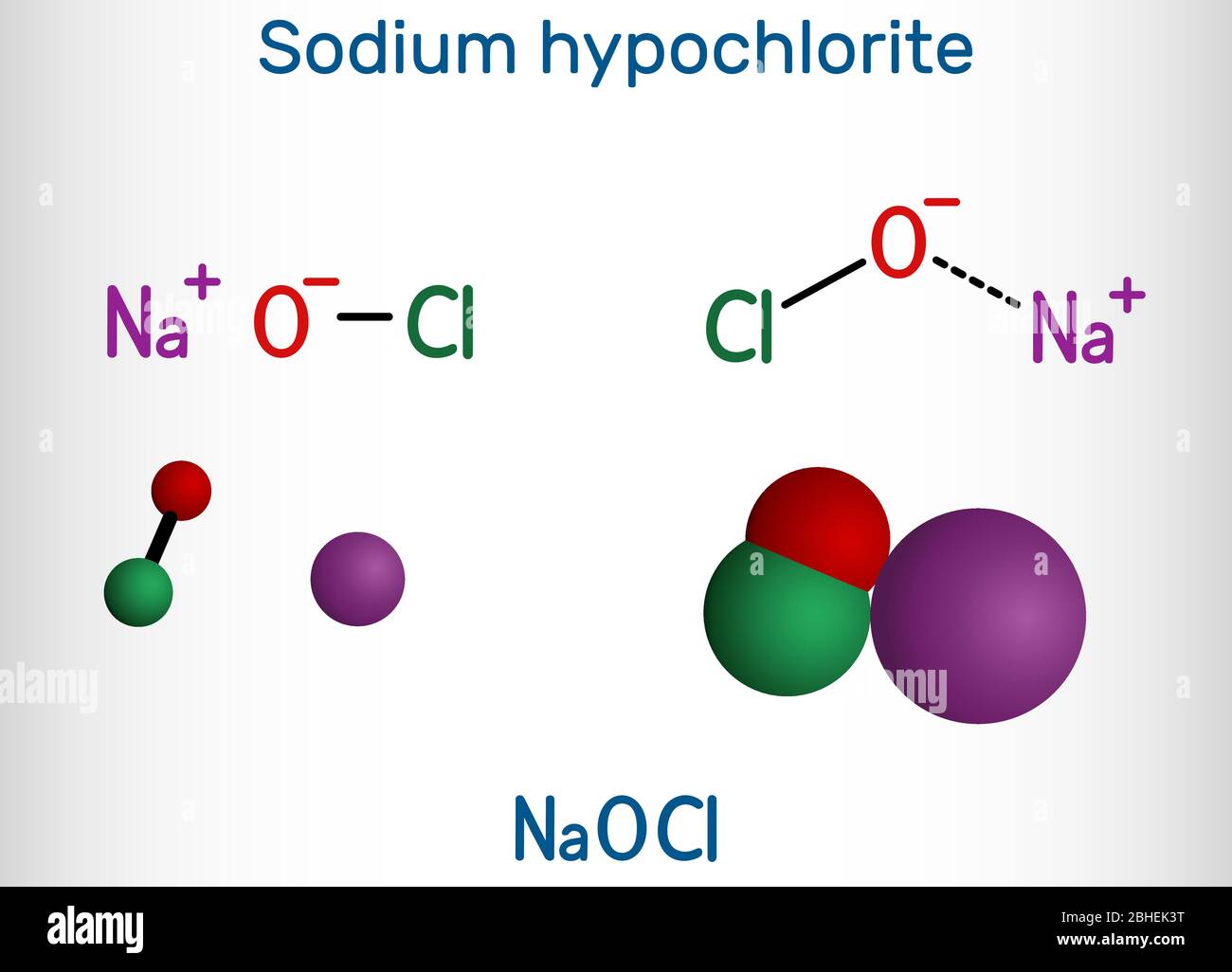
Sodium hypochlorite, NaOCl molecule. It contains a sodium cation and a hypochlorite anion. It is used as a liquid bleach and disinfectant. Structural Stock Vector Image & Art - Alamy

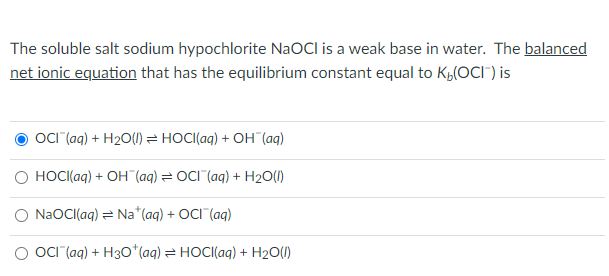
Sodium Hypochlorite Pentahydrate Crystals (NaOCl·5H2O): A Convenient and Environmentally Benign Oxidant for Organic Synthesis | Organic Process Research & Development

Dissociation of chloramine-T and NaOCl. (A) In the presence of water... | Download Scientific Diagram
SciELO - Brazil - Mechanism of action of sodium hypochlorite Mechanism of action of sodium hypochlorite

Sodium Hypochlorite (NaOCl) Molecule. Aqueous Solution Is Known As (liquid) Bleach. Skeletal Formula. Royalty Free SVG, Cliparts, Vectors, And Stock Illustration. Image 150281295.
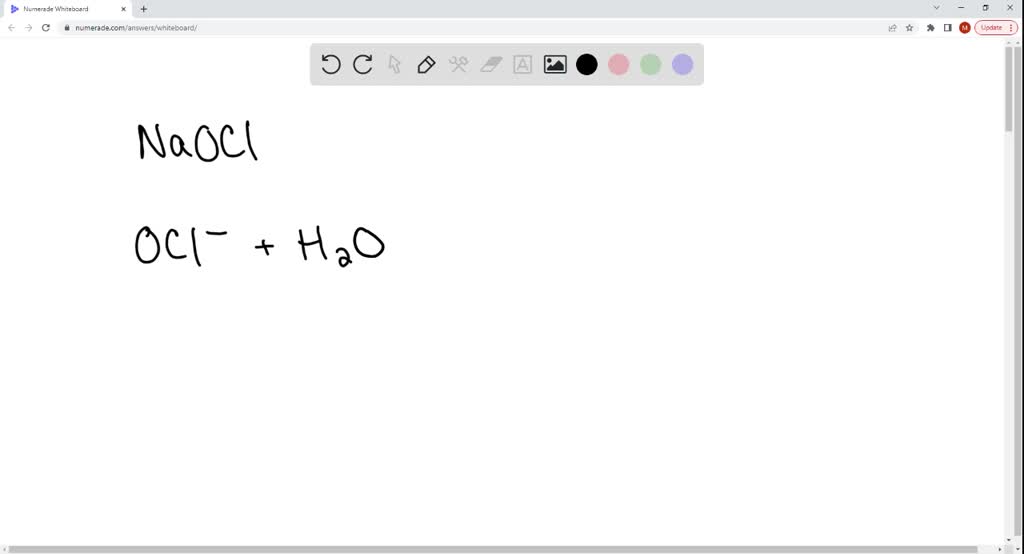

![Is NaOCl Acidic or Basic [Acids and Bases] - YouTube Is NaOCl Acidic or Basic [Acids and Bases] - YouTube](https://i.ytimg.com/vi/HXJWALr3BEY/maxresdefault.jpg)